As National Donate Life Month draws to a close, we have a guest blog from Uncle Jim. That’s the same Jim Myers who is off to Washington, D.C. to speak on our behalf, has more Facebook groups than I can count right now, has his own podcast, and is just always involved with kidney matters. We are lucky to have him on our team.

Jim has approached the same topic I wrote about several months ago, but his approach is much more detailed and more in-depth than the blog I wrote. Here is something kidney transfer recipients should keep in mind as you read today’s blog: Most kidney transplant patients experience hearing loss, especially at higher frequencies. Unfortunately, kidney transplantation may not significantly improve hearing problems.
Since Uncle Jim is so thorough, I’ve had to separate his guest blog into two blogs, so you know next week will be on the same topic. Take it away, Jim!
THE CONNECTION BETWEEN CKD AND HEARING LOSS

In my lifetime I have lost the hearing in my right ear. Recently, I discovered that my hearing loss may be connected to my 42 years of CKD/PKD, so I wanted to share what I have learned. I did a broadcast on Friday, March 8, 2024 on Hearing Loss and Kidney Disease. Here are some of my thoughts.
According to experts, there is a connection kidney disease and loss of hearing. (Nature.com)
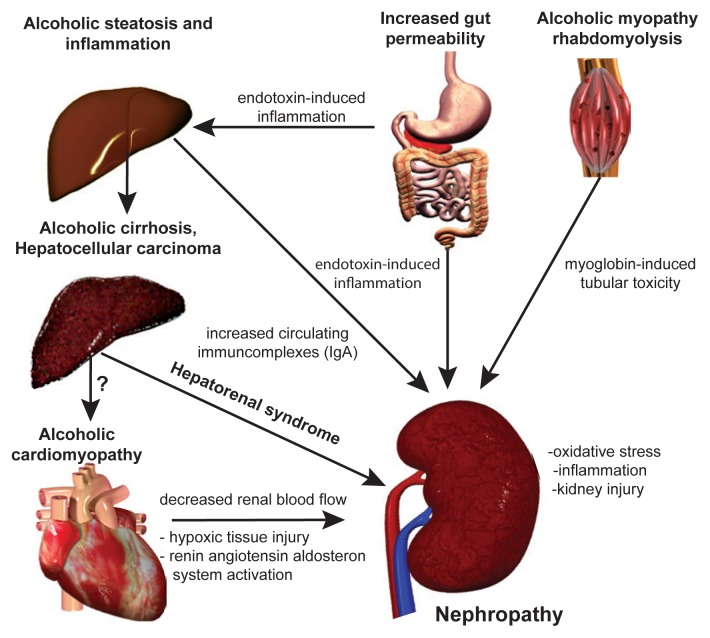
There are nearly 1.6 billion people that suffer from hearing loss & it is the third-leading cause of disability worldwide. Chronic kidney disease (CKD) is also a common condition that is associated with adverse clinical outcomes and high health-care costs. It affects 15% of US adults & 37 million x are estimated to have chronic kidney disease.
The question is whether or not there is a connection between the two. The answer appears to be yes. According to experts, the kidneys and the hearing organs share a common morphogenetic (same cells, tissue & genetic structure) origin and rely on similar biological structures (for example, cilia) and processes (for example, specialized cellular transport mechanisms) to function. So, the same Genetic Abnormalities that cause CKD can also cause hearing loss, and vice versa.
The NIH states,” Inadequate excretion of metabolic waste products by the kidneys results in circulation of these toxic materials in the body. This can cause damage to tissues and organ systems including the auditory system which can lead to hearing loss.” According to Nature.com, “A strong, graded and independent relationship exists between kidney function and the risk of hearing loss; the highest risk is observed in patients on haemodialysis, but kidney transplant recipients and people with mild CKD are also at increased risk.” Because tissue in our ear is substantially similar to the tissue in our kidneys, the toxic build up that damages kidney tissue also is capable of damaging inner ear tissue.
This appears to be confirmed by a 2010 study in Australia, that not just specific kidney diseases, but kidney disease in general can cause hearing loss in kidney patients. “This study examined the medical records of 2,564 people aged 50 and over, 513 of whom had moderate chronic kidney disease. Some 54.4% of all the patients with chronic kidney disease had some degree of hearing loss, as compared to only 28.3% of those who had no kidney problems.” Even more interesting, 30% of the CKD patients had a severe hearing loss compared to just 10% in those patients without CKD.”
The study concluded, “The link can be explained by structural and functional similarities between tissues in the inner ear and in the kidney. Additionally, toxins that accumulate in kidney failure can damage nerves, including those in the inner ear.” Also, some treatments for kidney ailments are ototoxic, meaning they cause hearing loss.”
As stated earlier, this is readily found in patients that are on hemodialysis. Experts suggest that infants, children and adults with malformation or dysfunction of their hearing organs should be evaluated for the presence of malformation or dysfunction of their kidneys, and people with kidney disease should have their hearing checked for loss.
Some types of kidney diseases are mentioned more prominently than others in the literature as causes of hearing loss and if you have one of these diseases you may wish to have your hearing checked as well as your kidney function. These diseases include:
• Alport’s Syndrome
• Polycystic Kidney Disease
• Meniere’s Disease
Many people with Alport’s Syndrome have problems with their ears and eyes. Alport syndrome is a rare inherited disorder that damages the tiny blood vessels in the kidneys. It can also cause hearing loss and eye problems. Alport syndrome is an inherited form of kidney inflammation (nephritis). It is caused by a defect (mutation) in a gene for a protein in the connective tissue, called collagen. The disorder is rare. There are three genetic types:
• X-linked Alport syndrome (XLAS) — This is the most common type. The disease is more severe in males than in females.
• Autosomal recessive Alport syndrome (ARAS) — Males and females have equally severe disease.
• Autosomal dominant Alport syndrome (ADAS) — This is the rarest type. Males and females have equally severe disease.
The frequency in which hearing loss appears with Alport’s is striking. Studies show that, approximately, 70% of patients with AS suffer from progressive sensorineural hearing loss. Over time, Alport syndrome also leads to hearing loss in both ears. By the early teens, it is more common in males with XLAS, though in females, hearing loss is not as common and happens when they’re adults. With ARAS, boys and girls have hearing loss during childhood. With ADAS, it occurs later in life. Hearing loss usually occurs before kidney failure. Approximately 80% of males with X-linked Alport syndrome (XLAS) develop hearing loss during their lifetime, often by their teens. Hearing loss in females with XLAS is less frequent and occurs later in life, although about 40% will experience hearing loss.
Studies have shown that Polycystic Kidney Disease can cause hearing loss. One study in particular found a family with ADPKD associated with bilateral sensorineural deafness in a pedigree of four affected members in four generations.

Gail here. I found myself wanting to read more, but this blog is already longer than usual. Keep yourself primed for the remainder of Uncle Jim’s guest blog next week.
By the way, have you listened to Uncle Jim interview me last Friday night? Here’s the YouTube of it:
Until next week,
Keep living your life!